Multiple Choice Questions
Multiple Choice QuestionsIn the brown ring complex [Fe(H2O)5(NO)]SO4, nitric oxide behaves as
NO+
neutral NO molecule
NO-
NO2-
A.
NO+
In Fe-complexes, NO behaves as NO+.
On passing 'C ampere of current for time 't' sec through 1 L of 2 (M) CuSO4 solution (atomic weight of Cu= 63. 5), the amount 'm' of Cu (in gram) deposited on cathode will be
m = Ct / (63.5 x 96500)
m = Ct / (31.25 x 96500)
m = (C x 96500) / (31.25xt)
m = (31.25 X C x t) / 96500
Which one of the following methods is used to prepare Me3COEt with a good yield?
Mixing EtONa with Me3CCl
Mixing Me3CONa with EtCl
Heating a mixture of (1:1) EtOH and Me3COH in the presence of conc. H2SO4
Treatment of Me3COH with EtMgI
Upon treatment with I2 and aqueous NaOH, which of the following compounds will form iodoform?
CH3CH2CH2CH2CHO
CH3CH2COCH2CH3
CH3CH2CH2CH2CH2OH
CH3CH2CH2CH2(OH)CH3
Upon treatment with Al(OEt)3 followed by usual reactions (work up), CH3CHO will produce
only CH3COOCH2CH3
A mi xture of CH3COOH and EtOH
only CH3COOH
only EtOH
Which one of the following properties is exhibited by phenol?
It is soluble in aq. NaOH and evolves CO2 with aq. NaHCO3,
It is soluble in aq. NaOH and does not evolve CO2 with aq. NaHCO3
It is not soluble in aq. NaOH but evolves CO2 with aq. NaHCO3
It is insoluble in aq. NaOH and does not evolve CO2 with aq. NaHCO3
The basicity of aniline is weaker in comparison to that of methyl amine due to
hyperconjugative effect of Me-group in MeNH2
resonance effect of phenyl group in aniline
lower molecular weight of methyl amine as compared to that of aniline
resonance effect of - NH2 group in MeNH2
Identify the method by which Me3CCO2H can be prepared.
Treating 1 mole of MeCOMe with 2 moles of MeMgI
Treating 1 mole of MeCO2Me with 3 moles of MeMgI
Treating 1 mole of MeCHO with 3 moles of MeMgI
Treating 1 mole of dry ice with 1 mol of Me3CMgI
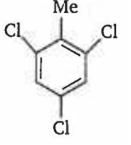
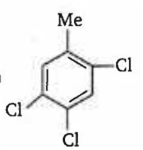
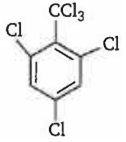
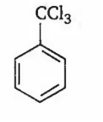
By passing excess Cl2 (g) in boiling toluene, which one of the following compounds is exclusively formed?