 Multiple Choice Questions
Multiple Choice QuestionsThe role of copper diaphragm in Whytlaw-Gray's method is
preventing the corrosion of electrolytic cell
preventing the mixing of H2 and F2
as anode
as cathode
B.
preventing the mixing of H2 and F2
In Whytlaw-Gray's method for preparation of fluorine, the copper diaphragm is used to prevent the mixing of H2 and F2 liberated at cathode and anode respectively.
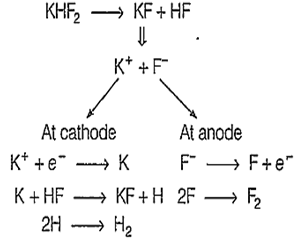
Reactions in the electrolytic cell-
Liquid X is used in bubble chamber to detect neutral mesons and gamma photons. Then, X is
He
Ne
Kr
Xe
Which of the following is not added durig the extraction of silver by cyanide process?
NaCN
Air
Zn
Na2S2O3
Which one of the following gives Prussian blue colour?
Fe2[Fe(CN)6]
Na4[Fe(CN)6]
Fe3[Fe(CN)6]3
Fe4[Fe(CN)6]3
The products formed, in the reaction of phenol with Br2 dissolved in CS2 at 0°C are
o-bromo, m-bromo and p-bromophenols
o-bromo and p-bromophenols
2,4,6-tribromo and 2,3,6-tribromophenols
2,4-dibromo and 2,6-dibromophenols
Example of a biodegradable polymer pair is
nylon-6, 6 and terylene
PHBV and dextron
bakelite and PVC
PET and polyethylene
The number of hydrogen bonds between guanine and cytosine and between adenine and thymine in DNA is
1, 2
3, 2
3, 1
2, 1
