 Multiple Choice Questions
Multiple Choice QuestionsOptical isomerism is exhibited by (ox= oxalate anion; en= ethylenediamine).
cis-[CrCl2(ox)2]3-
[Co(en)3]3+
trans-[CrCl2(ox)2]3-
[Co(ox)(en)2]+
A.
cis-[CrCl2(ox)2]3-
B.
[Co(en)3]3+
D.
[Co(ox)(en)2]+
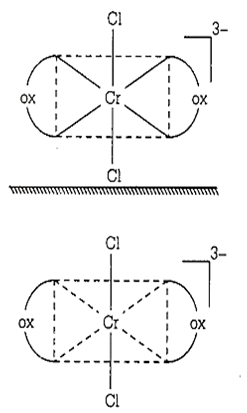
In the above figure, trans-[CrCl2(ox)2]3- isomer-optically inactive (superimposable mirror images and plane of symmetry).
cis-[CrCl2(ox)2]3-, [Co(en)3]3+ and [Co(ox) (en)2]+ exhibited optical isomerism.
In a mixture, two enantiomers are found to be present in 85% and 15% respectively. The enantiomeric excess (ee) is
85%
15%
70%
60%
In the Lassaigne's test for the detection of nitrogen in an organic compound, the appearance of blue coloured compound is due to
ferric ferricyanide
ferrous ferricyanide
ferric ferrocyanide
ferrous ferrocyanide
Extraction of gold (Au) involves the formation of complex ions X and Y.
Gold ore HO- + X Y + Au
X and Y respectively are
Au(CN) and Zn(CN)
Au(CN) and Zn(CN)
Au(CN) and Zn(CN)
Au(CN) and Zn(CN)
The atomic number of cerium (Ce) is 58. The correct electronic configuration of Ce3+ ion is
[Xe]4f1
[Kr]4f1
[Xe]4f13
[Kr]4d1
Sulphuryl chloride (SO2Cl2) reacts with white phosphorus (P4) to give
PCl5, SO2
OPCl3, SOCl2
PCl5, SO2, S2Cl2
OPCl3, SO2, S2Cl2
For the reaction A +2B → C, the reaction rate is doubled, if the concentration of A is doubled. The rate is increased by four times when concentrations of both A and B are increased by four times. The order of the reaction is
3
0
1
2
Cold ferrous sulphate solution on absorption of NO develops brown colour due to the formation of
paramagnetic [Fe(H2O)5(NO)]SO4
dimagnetic [Fe(H2O)5(NO)]SO4
paramagnetic [Fe(H2O)5(NO3)][SO4]2
diamagnetic [Fe(H2O)4(SO4)]NO3
Suppose the mass of a single Ag-atom is m. Ag metal crystallises in fcc lattice with unit cell of length a. The density of Ag metal in terms of a and m is
